

FDA and Merck have known about these harms for 25 years and yet failed to warn pediatricians or parents
Background and context
When Merck’s M-M-R II was licensed in 1978, a proper clinical trial of its safety was never conducted. From a prior ICAN demand to the FDA, we know that the safety was only assessed in 834 children for a period of 42 days in trials that had no placebo control. Further, within the first 42 days following administration of the vaccine, in one of the largest trials for this vaccine, 63% of the children experienced gastrointestinal illness and 42% had upper respiratory illness. Yet the FDA approved the product anyway.
Ignoring troubling safety signals appears to be a pattern with the FDA. Fast forward to 1995 – following licensure of another Merck vaccine, VARIVAX (attenuated, live, varicella, a.k.a. chickenpox, vaccine), the FDA required a post-licensure safety study which Merck conducted from June 1, 1995 through February 5, 1997. In 2019, ICAN, though its attorneys, filed a Freedom of Information Act request for that post-licensure study, which the FDA finally produced a few months ago.
The discovery of the VARIVAX post-licensure study
Merck’s Phase-IV post-licensure study included 34,655 children 12-23 months old and 51,463 children 2 to 12 years of age who were injected with VARIVAX. About 60% of these children aged 12-23 months and 17% of these children aged 2 to 12 years also received the improperly tested M-M-R II vaccine, in addition to other vaccines, at the same time they received VARIVAX. The study found several troubling safety signals.
The study found more than 60 conditions including allergic reactions, alopecia, arthritis/arthralgia, and gastroenteritis that were significantly elevated following VARIVAX vaccination. This suggests this vaccine, soon after administration, may lead to a wide range of reactions that in many cases are worse than the usual chickenpox rash.
But then there was this additional bombshell that, as far as we know, has never been disclosed to the public. The post-licensure study shows that there are significantly more adverse events among children receiving both VARIVAX and M-M-R II compared to those receiving VARIVAX alone (summary table below).
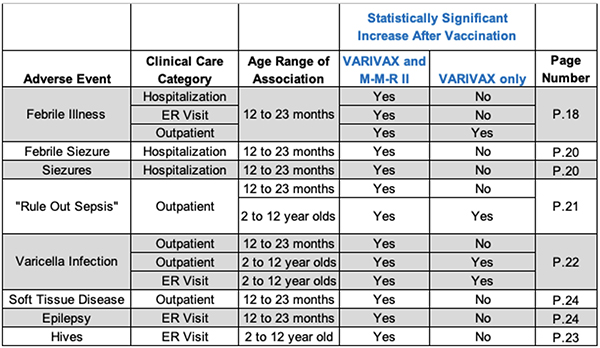
Every parent should be horrified by this information. To be clear: what is notable here is that the FDA knew, as early as the first report of this study in September 1997, that the likelihood of an adverse event in children 12-23 months old and 2-12 years old appears significantly increased if the child receives VARIVAX at the same time as the M-M-R II vaccine.
The study also noted: “It should be born in mind that concomitant vaccination with MMR could be a marker for vaccination with several other vaccines simultaneously.” (p. 19).
This raises multiple red flags. The FDA and Merck know that VARIVAX is linked with increased rates of troubling adverse events. And the FDA and Merck know that the CDC schedule increases the rate of harms given that it calls for concomitant administration of VARIVAX and MMR (and potentially “several other vaccines”)!
So, what did the FDA do about it? Did it reevaluate the licensure of VARIVAX? No. Did it reevaluate the licensure of MMR, M-M-R II, or MMRV? No. Did it, at the very least, immediately recommend that VARIVAX and M-M-R II no longer be given concomitantly? No. And it still has not done so, 25 years later.
The FDA had a legal and moral obligation to release this information to the public so that parents could make informed decisions. Of course, as we have seen repeatedly, the FDA is loath to admit it has made a mistake. So, this report was essentially buried for 25 years until ICAN finally took action to obtain a copy.
More than two decades later, the FDA has not even bothered to update the package inserts for M-M-R II to disclose these harms!
In fact, despite the results of this study, the package insert for VARIVAX today explicitly claims that administering the two together is perfectly safe: “VARIVAX may be administered concomitantly with M-M-R II.”
This is yet another example of deception on the American people and why federal health authorities should have no role in recommending, let alone mandating, any product, but especially any product that can have adverse effects. Once federal health authorities become partners in the success of a vaccine product, including having their reputations tied to its success and being the party responsible for defending against claims it causes harm, admitting issues or mistakes becomes a devastating proposition to the very same health authorities that are supposed to ensure vaccine safety. This conflict is intractable and destructive.
ICAN will never stop fighting to bring you the truth and making sure that every last government official that has failed to safeguard the health of our children is held accountable.
