

In case you missed it, after over a year of legal demands and litigation, ICAN recently announced that it had obtained data from v-safe, the CDC’s new smartphone-based program to track adverse reactions to the COVID-19 vaccines.
As part of those legal efforts, ICAN’s legal team also tracked down a copy of the CDC’s January 28, 2021 v-safe protocol which reveals something incredible. The v-safe protocol lists a series of 15 “Adverse Events of Special Interest” but, unbelievably, the v-safe program itself does not even track these adverse events!
Worse yet, the next version of the v-safe protocol, dated May 20, 2021, again lists the same series of 15 “Adverse Events of Special Interest” but, unbelievably, the v-safe program still did not track these adverse events!
These adverse events were indeed serious and included the following:
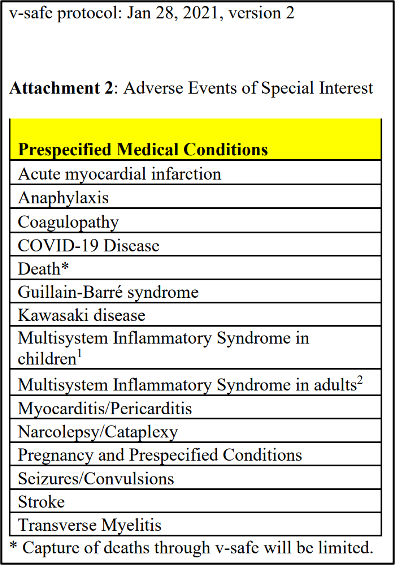
Instead of asking v-safe participants whether they experienced any of the above-listed serious adverse events, the v-safe system only asked about minor and generalized reactions such as “Chills,” “Headache,” “Fatigue or tiredness,” and “Vomiting.” See the ICAN v-safe Dashboard for a list of what was tracked.
If users did in fact want to report one of the known “Adverse Events of Special Interest,” they would have to type it into a free-text field in v-safe that had a limited character count. ICAN continues to litigate in order to obtain the data from the v-safe free-text fields.
This was an incredible decision by the CDC that, in developing a program specifically designed to track adverse events to the COVID-19 vaccines, it chose not to include a single one of the serious adverse reactions it specifically knew to be on the lookout for in its list of pre-populated adverse events.
V-safe did not even specifically track myocarditis or other cardiac events despite the CDC’s acknowledgment of this well-known adverse event! One might wonder whether this choice was intentional.
As ICAN has said before, the fight for transparency with respect to this data is not over. Stay tuned because ICAN will make public the data from the free-text fields, which will hopefully give a clearer picture of how frequent those “Adverse Events of Special Interest” really were.
